SHINE BRIGHT LIKE A DIAMOND
Let’s sparkle this Spring.








MARK YOUR CALENDARS

What are the 4Cs of diamond quality?
The GIA 4Cs of diamond quality helps you understand a diamond’s characteristics as well as its value and price.
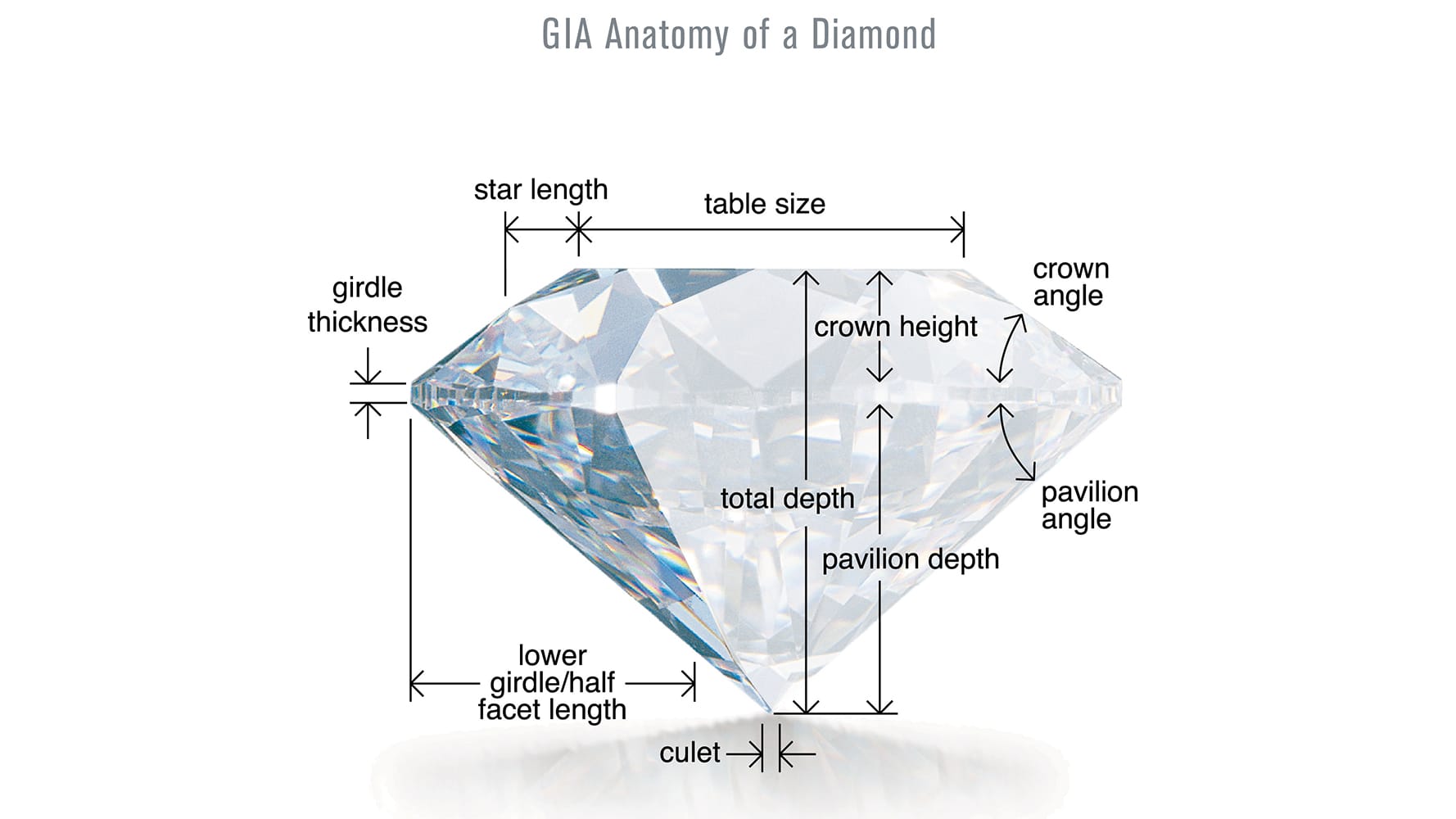
- Cut is perhaps the most crucial of the 4Cs. Cut does not refer to a diamond’s shape, but rather to the quality of workmanship (proportion and arrangement of facets) for round brilliant diamonds.* The quality of a diamond’s cut determines the amount of brilliance, sparkle and fire that a diamond shows. Grades range from ‘Excellent’ to ‘Poor.’

The diamonds in this image show Poor, Good and Excellent cut grade from left to right.

- Color measures a diamond’s absence of color. This is because most “colorless” or “white” diamonds actually contain trace amounts of yellow, brown or gray color. The less color in a diamond, the more desirable and valuable it is. There are 23 color grades on the D-to-Z scale, with D meaning that a diamond has no detectable color at all and Z meaning that a diamond has “light” color. Any diamond beyond the Z color grade is a fancy color diamond and is evaluated on a different color scale.
- Color grade impacts the price of a stone, but differences of one to three color grades are not easily detectable to untrained eyes. Diamonds graders evaluate diamonds face-down in special environments to see subtle color differences.
Tip: D, E and F grade diamonds tend to be extremely rare and valuable. G and H diamonds are typically considered good value. Color becomes more visible in the I grade and below.

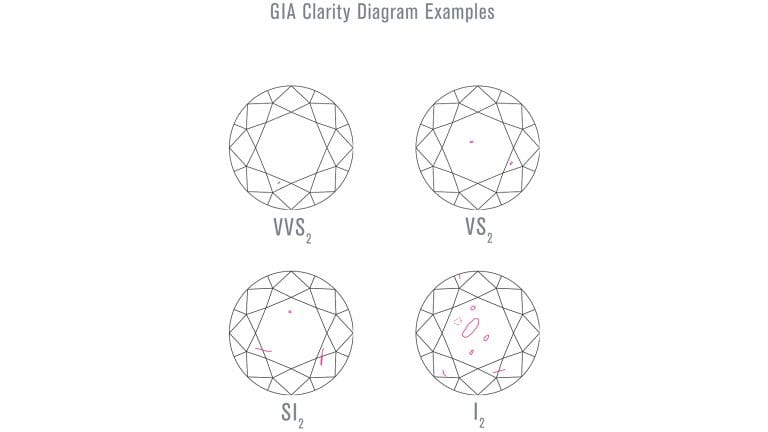
- Clarity measures the amount, size and placement of internal ‘inclusions’ and external ‘blemishes.’ Inclusions include small crystals or fissures within the diamond. Blemishes include chips. Grades range from ‘Flawless,’ which means a diamond has no visible imperfections at 10x magnification, to ‘Included,’ which means a diamond contains a significant number of imperfections. Diamonds with grades down to VS2 (Very Slightly Included) or SI1 (Slightly Included) do not typically have eye-visible inclusions. These diamonds can be good value. Diamonds I1 (Included) or lower have inclusions that are easily seen and can appear less attractive; some of these inclusions might also impact the diamond’s durability.
Tip: Brilliant-cut diamonds show clarity characteristics less than step cut diamonds do, because the pattern of the facet arrangement obscures inclusions better. If you want to buy a step cut diamond (such as an emerald cut), you may have to go higher in color and clarity than with a brilliant-cut diamond.

- Carat refers to a diamond’s weight. Generally speaking, the higher the carat weight, the larger the diamond appears and the more valuable the stone. How large a diamond appears also depends on its proportions. For example, a one-carat diamond that is wider but has shallow proportions will appear larger than a one-carat diamond with excellent proportions.

Tip: Diamond prices go up exponentially as carat weight increases. They increase more at certain “magic sizes,” such as 0.5, 1.0, 1.5, 2.0 carats etc. Buying right below a “magic size,” such as purchasing a 0.95 carat diamond instead of a 1.0 carat diamond, can save money without making much of a difference in visual impact.

Diamond fluorescence is a fascinating phenomenon where diamonds glow when exposed to long-wave UV rays. It can sometimes increase or decrease the value of a diamond. It can also affect diamond appearance—or not.
Fluorescence is not a grading factor like the GIA 4Cs (color, clarity, cut, and carat weight), but it is an identifying characteristic. GIA Diamond Grading Reports and Diamond Dossiers describe a diamond’s fluorescence by its intensity under long-wave UV light (None, Faint, Medium, Strong and Very Strong). If the fluorescence is Medium, Strong, or Very Strong, the color of the fluorescence will be noted.

Diamond fluorescence is neither good nor bad. Some people find fluorescent diamonds beautiful and fascinating—others may not. Opinions range across the spectrum.
CARING FOR A DIAMOND

Diamonds are remarkably durable, resist scratching (except by other diamonds) and maintain their brilliance over time. But diamonds aren’t indestructible. They can be chipped by a sharp blow, become loose or lost in a weakened setting, or be damaged by contact with other diamonds. Wear diamond jewelry with care. Store it in padded boxes or soft bags separate from other jewelry. Clean your jewelry by wiping it with a lint-free cloth or with warm water, mild soap and a soft toothbrush, or by dipping it briefly in a commercial cleaning solution. Have your diamond jewelry periodically cleaned and its setting examined by a professional jeweler to maintain its beauty and integrity over time.

WHERE DO DIAMONDS COME FROM?

Diamond Formation
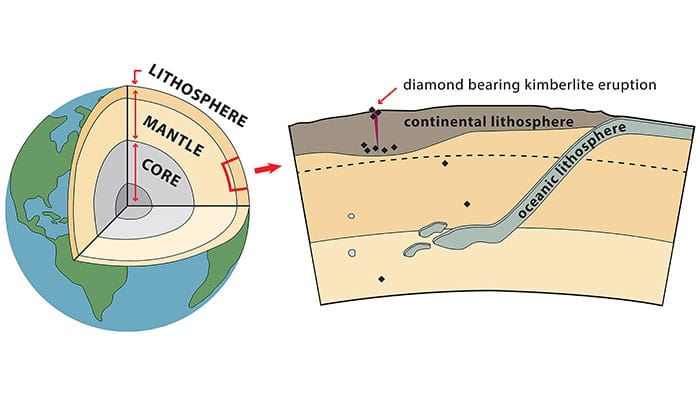
More than a billion years ago, 100 miles (161 km) or more beneath the earth’s surface, in a cauldron of extreme temperatures and high pressure, carbon atoms bonded tightly together. At temperatures higher than 2100oF (1150o C) and pressures 45,000 times greater than at sea level, crystals formed, resulting in the hardest natural mineral on Earth: diamond.
Diamonds remained hidden deep within the earth for hundreds of millions of years, until volcanic activity violently transported them upwards towards the earth’s surface in magma. Vertical rock formations, called “kimberlite pipes,” are remnants of these ancient volcanoes. Over time, erosion frees some of these rough diamonds from their host rock. Transported by rivers and streams, these diamond crystals end up in river gravel beds and silt often at great distances from their original source. Until the late 1800s, the world’s diamonds were found and collected from these alluvial beds.
Today, most diamonds are found in kimberlite pipes, which are the primary source of mined diamonds.
The discovery of a kimberlite pipe in South Africa in 1869 marks the beginning of the modern diamond industry. With it came the development of mining operations producing tens of millions of carats of rough diamonds each year – that includes a major discovery in Botswana in 1967, as well as other areas of Africa, Australia, Siberia and the Northwest Territories of Canada.
By the late twentieth century, the earth was no longer the only source for diamonds. In 1970, General Electric began creating diamonds in a laboratory and the first gem-quality laboratory-grown diamond were sold in 1984. With advances in technology, man-made diamonds have become more common and harder to detect.
LAB GROWN DIAMONDS

Laboratory-grown diamonds are increasingly available in greater quantities, higher quality and new colors at price points that many find attractive. It is no wonder then that laboratory-grown diamonds are a growing presence in the marketplace.
Laboratory-grown diamonds have essentially the same chemical, optical and physical properties and crystal structure as natural diamonds. Like natural diamonds, they are made of tightly-bonded carbon atoms. They respond to light in the same way and are just as hard as natural diamonds. The main differences between laboratory-grown and natural diamonds lie in their origin. Think of it this way: laboratory-grown diamonds are like ice from your refrigerator, while natural diamonds are like ice from a glacier. They are both ice, although their formation stories and the age of each are very different.
Natural diamonds formed millions to billions of years ago in Earth’s mantle then were explosively carried by kimberlite and lamproite volcanoes to Earth’s surface, often carrying fascinating inclusions within them. The oldest laboratory-grown diamonds are decades old; they are created in laboratories or large factories, most commonly using the High-Pressure, High-Temperature (HPHT) method or the Chemical Vapor Deposition (CVD) method. Laboratory-grown diamonds appear identical to natural diamonds to the unaided eye and typically require testing by a laboratory with advanced instruments to be identified.

This laboratory-grown diamond (left) and natural diamond (right) cannot be told apart using the unaided eye.
Laboratory-Grown Diamond Timeline
- 1950s: Union Carbide produces the first Chemical Vapor Deposition (CVD) diamonds in 1952. Others produce diamonds using the high pressure, high temperature (HPHT) method soon after. These diamonds are used for industrial purposes, such as in telecommunications and laser optics and as abrasives and more.
- 1970s: General Electric researchers create the first gem-quality laboratory-grown diamonds. They are of high enough clarity and large enough size to be used in jewelry. GIA scientists publish the first scientific study of laboratory-grown diamonds in 1971.
- Mid-1980s: Manufacturers grow commercial quantities of gem-quality laboratory-grown diamond crystals. These lab-created diamonds are initially mostly small and yellowish or brownish in color, but their quality improves over the ensuing decades.
- 2000s: Gem-quality diamonds are created using the chemical-vapor deposition (CVD) method, which requires lower pressures and temperatures than the HPHT method.
- Mid-2010s: Colorless laboratory-grown diamonds are available in the jewelry market in commercial quantities. Both HPHT and CVD continue to be popular methods of laboratory-grown diamond production.

Different growth methods produce different diamond crystal shapes. Seen here are a CVD (left), HPHT (middle) and natural diamond crystal (right). The CVD diamond is edged in non-diamond carbon from the growth process. The natural octahedral diamond is covered in etch marks called trigons.
How are laboratory-grown diamonds made?
There are two main processes used to create laboratory-grown diamonds:
1. High Pressure, High Temperature
With this method, laboratory-grown diamonds are produced using high-pressure, high-temperature conditions similar to what natural diamonds experience in the earth. HPHT diamond growth occurs at pressures of 5–6 GPa (roughly equivalent to the pressure exerted by a commercial jet airplane if balanced on the tip of a person’s finger) and at temperatures of 1300–1600°C.
Lower-quality diamonds, whether natural or laboratory-grown, can also be put through the HPHT process to improve color. In addition to making diamonds more colorless, this process can also be used to change the color of diamonds to pink, blue or yellow. The diamond would then be called a “treated” diamond.
DIAMOND FUN FACTS













APPRAISALS….
Protect the pieces you love! Let us help you sort through and appraise your jewelry, from rings to watches and everything in-between. Whether you need a new insurance appraisal, update an old one, or itemized list for an estate appraisal, we do it all!
Inquire more information with an associate.
WE BUY GOLD, PLATINUM, SILVER, AND ESTATE JEWELRY
While the market is us, go through your jewelry box and find those unworn and/or broken pieces and turn it in for a check or store credit. Or let us help recreate a new piece that you will wear, using that original gold credit toward it.
HAPPY APRIL AND HOPE TO SEE YOU SOON!
Comments are closed





